
BIOREADER® 7000 -E
Our Bioreader® counts 100% of the filter area! No losses due to the exclusion of the outer border/filter region.

The BIOREADER® 7000 -E is the best choice to evaluate enzymatic Elispot assays (single or dual color), Plaque/FOCI assays or other assays in 96 well plates.
Verified and validated in international studies.
Model variants:
The Alpha is optimized for one specific plate type. This model is commonly used for routine applications, for example when assisting in TB, COVID or CMV diagnostic Elispot measurements.
The Beta can be used for all other kinds of 96 well plates, incl. wells with small volume, transparent, black and white plates with clear bottom and transparent or white Microfilter plates. This model can also count 2 different immunohistochemical or enzymatic colors per well.
This Bioreader® is able to quantify visible stain. For instant CV, DAB (3,3′-Diaminobenzidine), Substrates TMB,PCIP/NBT,AEC as well as Silver Staining.
- Affordable Bioreader® 7000 –E enzymatic assay reader
- Brand-new generation 7 makes it significantly faster and more precise regarding positioning.
- Approved and verified in worldwide collaborative studies: > 300 Bioreader® units sold worldwide.
- Upgradable: On-site installation of other software packages.
- Measure Protocol development assistance: includes Spot Profiling to automatically generate optimized measure protocols.
- Verified measure programs.
- Front loader, automatic door.
- Dual tele-centric illumination.
- Interactive training program.
- Export to Excel/Word/Power Point.
- LIMS/LIS import and export capabilities.
- High contrast/low noise cameras from 5 - 41 MPixel available.
- Incl. computer and monitor.
- Optional automation with plate 'feeding system'.
- DQ/IQ/OQ/PQ documentation possible.
- 12 months warranty.
- EC declaration of conformity and EMC certificate.
- Innovative Bioreader® and EazyReader® software combines ‘easy of use’ and versatility and flexibility.
- Scan, analyze and overlay live time.
- Creates up to 7 images for each well simultaneously during the scan.
- ‘Profiling’ app helps to create user independent measure protocols for Elispot beginners and references for experts.
- Verified in collaborative studies.
- Export options and reports with all scalable images and results even in one file.
- Customer specific export and report templates.
- Video clips and content specific help files.
- Qualified installation and training with each instrument.
- On-site or internet remote services and support.
- ‘Classified’ measure protocols ‘history’ tracking and comparison tools.
- User specific plates, Studies/projects, plate layouts/designs and measure protocols prevent from mix-up.
- More accurate ‘cytokine quantification’ based on the patented ‘photometric’ dual illumination system.
- Optional software features:
- 'Routine’ software package: Ooptimized applications for commonly used operations, only presents the icons you require for the specific job, quickly read a plate and quickly review, Q.C. and release the results.
- 21 CFR part 11 based software module available.
Dimension
Weight

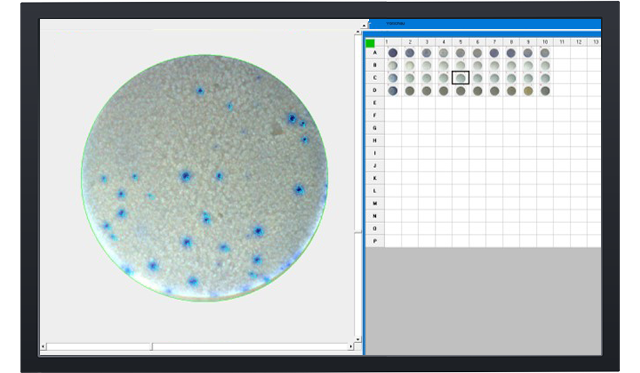
Elispot - Enzymatic single color
Blue, red, green or silver substrat Elispot.
Detection of antibodies. Enzyme-conjugated and precipitating substrate.
"The enzyme-linked immune absorbent spot (ELISpot) is a type of assay that focuses on quantitatively measuring the frequency of cytokine secretion for a single cell."
[https://en.wikipedia.org/wiki/ELISpot]
Why Elispot?
The cytokine Elispot assay is designed to enumerate cytokine secreting cells in single cell suspensions of lymphoid tissue, CNS tissue, bone marrow or preparations of peripheral blood mononuclear cells (PBMC).
The assay has the advantage of detecting only activated/memory T cells and the cytokine release can be detected at the single cell level, allowing direct determination of T cell frequencies.
The ELISPOT assay is an effective tool to enumerate antigen-specific T cells in the circulation of immunized humans and animals at much lower frequencies than possible with other currently available methods.
The ELISPOT assay has proven to be a sensitive and unique system to follow disease progression in human individuals or animals. Several studies have indicated that alterations in the frequency of cytokine pc in different compartments of the body adequately reflect changes in immune function.
The ELISPOT assay may be used to determine effects of drugs, chemicals or other compounds on cytokine secretion in vitro, thereby providing data on their putative modulatory effects on immune function in vivo
The ELISPOT assay is currently being used increasingly for the quantitative assessment of peptide reactive T lymphocytes from PBMC in infectious disease (3, 9) or in the course of vaccination trials aimed at the induction of tumor-specific T cells in patients with cancer.
Elispot may be used for Diagnosis of genetic defects,Allergic diseases,Autoimmune diseases,Transplantation,Cancer research,Acute inflammation,Acute infectious diseases and septic shock.

Elispot in 384 well microfilter plates
Small volume, higher throughput.

Elispot - Enzymatic dual color
Blue and red Elispot substrate (mixed color violet)
Dual cytokine secretion.

TCID50 viral assay
Cells were infected and covered with an overlay. Cells stained with CV survive. Unstained cells were killed by the virus.

Foci assay: total well view
"The focus forming assay (FFA) is a variation of the plaque assay, but instead of relying on cell lysis in order to detect plaque formation, the FFA employs immunostaining techniques using fluorescently labeled antibodies specific for a viral antigen to detect infected host cells and infectious virus particles before an actual plaque is formed."

Neutralization assay
"The plaque reduction neutralization test is used to quantify the titer of neutralizing antibodies for a virus. The serum sample or solution of antibodies to be tested is diluted and mixed with a viral suspension."
[https://en.wikipedia.org/wiki/Plaque_reduction_neutralization_test]




